
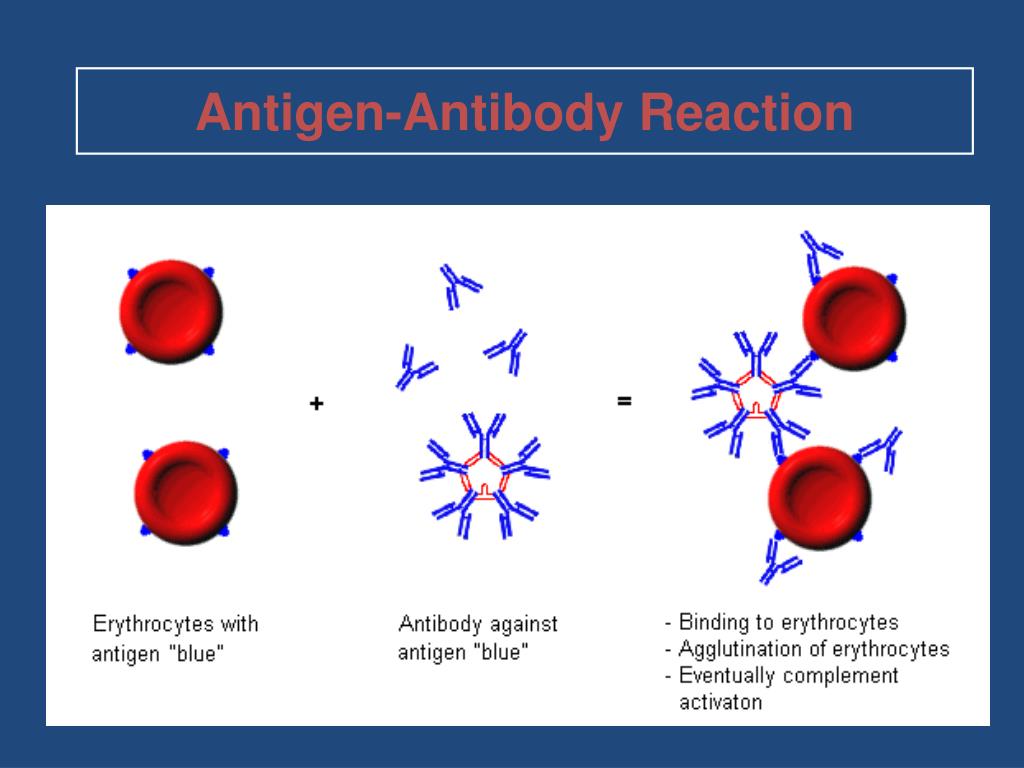
The third section introduces the different stages in the maturation of antibody specificity. A single paratope can bind to unrelated epitopes, and a single epitope can bind to unrelated paratopes.

Paratopes and epitopes define complementary regions of shape and charge rather than particular amino acid compositions. Each paratope has about 15 amino acids, of which about 5 contribute most of the binding energy for epitopes. Antibodies have a variable region of about 50 amino acids that contains many overlapping paratopes. The second section focuses on the paratope, the part of the antibody molecule that binds to an epitope. A change in any of those 5 key amino acids can greatly reduce the strength of antibody binding. However, only about 5 of the parasite's amino acids contribute to the binding energy. An antibody bound to an epitope covers about 15 amino acids on the surface of a parasite molecule. The surfaces of parasite molecules contain many overlapping antibody-binding sites (epitopes). The first section discusses antibody recognition. The molecular determinants of specificity and cross-reactivity define the nature of antigenic variation and the selective processes that shape the distribution of variants in populations.
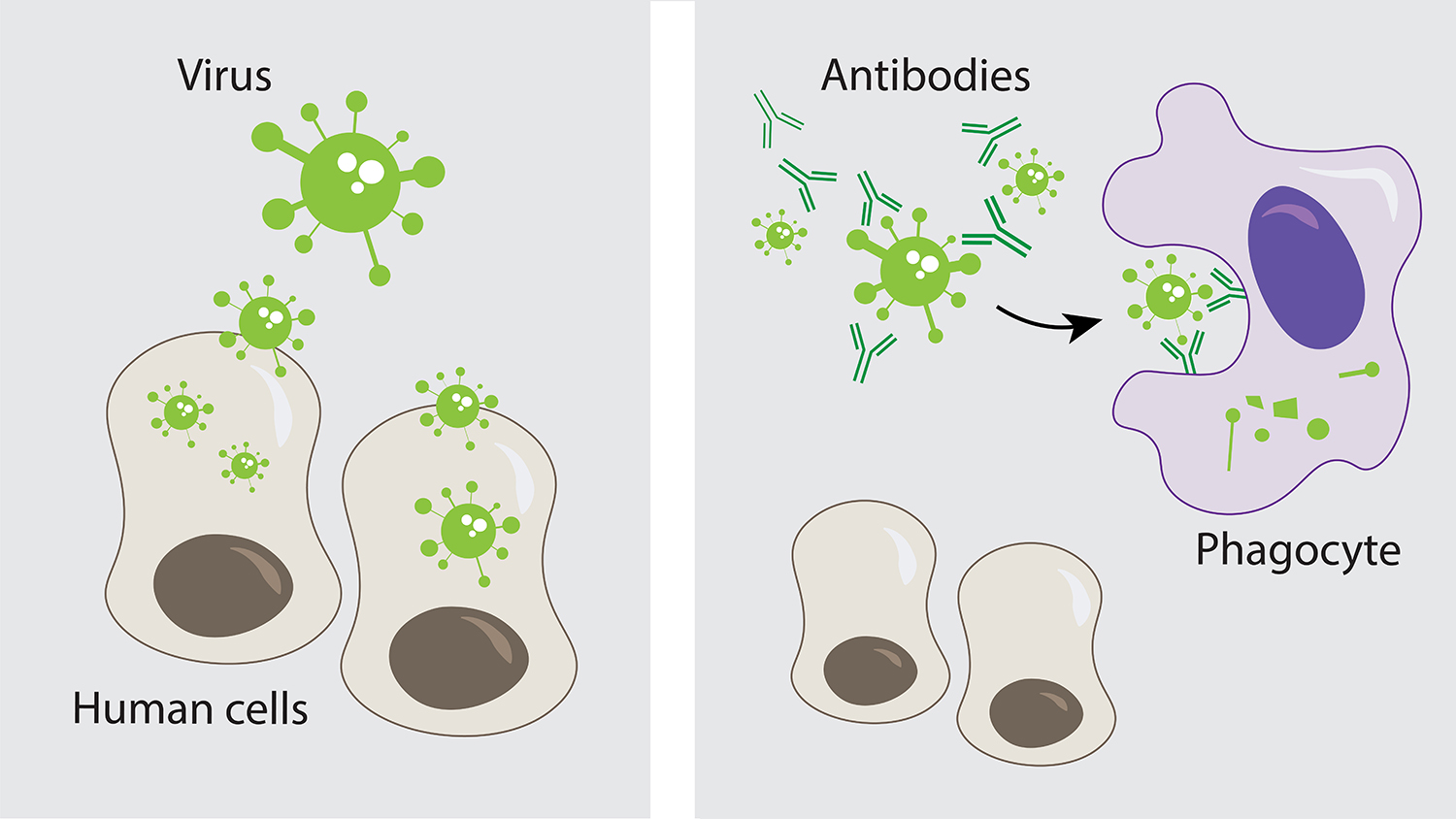
Cross-reactivity measures the extent to which different antigens appear similar to the immune system. Specificity measures the degree to which the immune system differentiates between different antigens. Two terms frequently arise in discussions of recognition. Circulating immune complexes may provide a reservoir of antibody with potential diagnostic and therapeutic applications.In this chapter, I describe the attributes of host and parasite molecules that determine immune recognition. Specificity analysis of one serum sample after dissociation revealed that the antibody detected an antigen common to SCCHN cell lines as well as melanoma, glioma, renal, and colon carcinoma cell lines. Only six of the untreated sera had detectable antibody reactivity against the autologous SCCHN cell line whereas following AD-U all 12 sera had enhanced IgG reactivity against autologous SCCHN. Twelve serum samples from eight patients were subjected to acid dissociation and ultrafiltration (AD-U).

Due to the low titer of autologous antibody reactivity in most sera, we sought to determine if dissociation of immune complexes through acidification and ultrafiltration of serum might enhance detectable antibody reactivity as has been done in previous studies in melanoma. Absorption analysis showed that this antigen was also expressed on 6 of 10 allogeneic SCCHN cell lines but not on autologous fibroblasts or on allogeneic melanoma cell lines. Analysis of higher titer sera from one patient revealed antibodies that define an antigen expressed on autologous tumor cells cultured from both the primary tumor (UM-SCC-17A) and from a metastasis (UM-SCC-17B). In 10 cases autologous antibody reactivity could be detected only in undiluted serum precluding further analysis. Autologous antibody reactivity (median titer of 1:4) was detected in sera from 24 of the patients tested. Serum antibody reactivity to squamous cell carcinoma of the head and neck (SCCHN) was evaluated in 41 autologous serum-tumor cell line combinations using the protein A hemadsorption assay.


 0 kommentar(er)
0 kommentar(er)
